CO2 fluxing and carbon assimilation by arc melts during magma–limestone interaction
Deegan F.M., M. Capriolo, V.R. Troll, F.A. Weis, S. Callegaro, S. Colucci, C. Freda, V. Misiti, L.E. Aradi, H. Skogby, H. Darmawan, H. Geiger (2025).
Chemical Geology, 704. https://doi.org/10.1016/j.chemgeo.2026.123264
Abstract
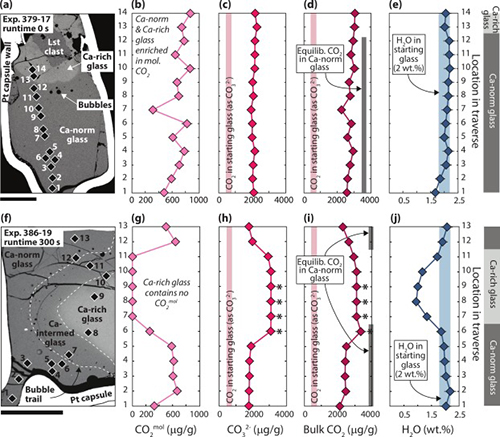
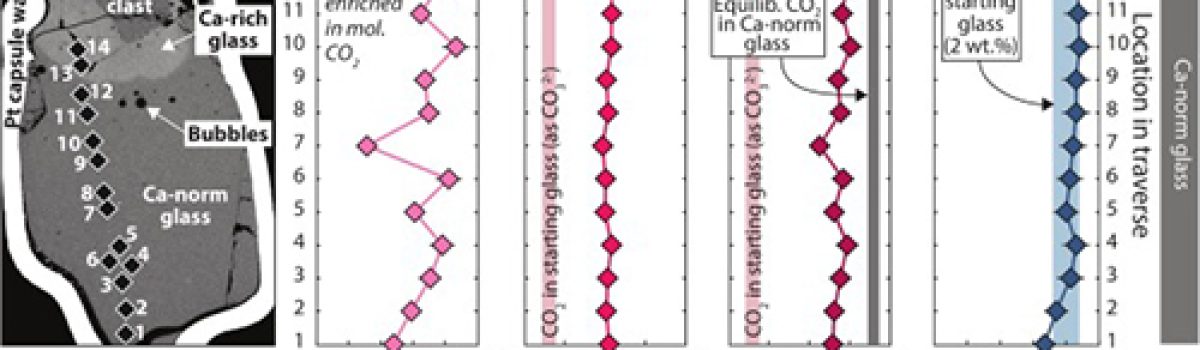
Reworking of limestone (CaCO3) by magma is an important source of carbon in volcanic arc emissions. However, while it is broadly understood that CO2 is liberated during magma–limestone interaction, the degassing behaviour of calcite in silicate melts is less well constrained. In this study, we carried out microspectroscopic analysis of volatiles within fluid inclusions and glass (former melt) in the products of short-term experiments simulating limestone assimilation in mafic arc melt (T = 1200 °C, P = 0.5 GPa, runtimes of 0 to 300 s). The experimental products consist of partly to wholly assimilated limestone xenoliths enveloped by CaO-rich silicate glass (reacting melt) that grades into mafic glass (host melt). Micro- to milli-metric sized fluid-filled bubbles permeate the experimental products. This study reveals that limestone assimilation induces extremely fast apparent diffusivity of CO2 (DCO2 ≳ 10−7 m2/s) through both the reacting melt and the host melt. Volatile saturation is thus quickly reached, triggering nucleation of bubbles mainly containing CO2 ± CO, CH4, N2, H2, and H2O. Crucially, we find that the host melt contains dissolved CO2 from limestone, despite showing no other compositional evidence for limestone assimilation. Mafic melts in volcanic regions underlain by limestone may therefore mobilise and transport more carbon than previously thought, with implications for eruptive behaviour, volcanic CO2 inventories, and long-term climate warming.


Devi effettuare l'accesso per postare un commento.